top of page

PUBLICATIONS
2016 - current
2026
M. Kim†, S. Y. Ahn†, S. Kim†, J. Won, D. Kim, and S. Y. Hong* (†Equally contributed)

2025
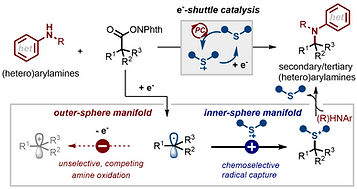
1. Cooperative Organosulfur/Photoredox Catalysis Enables Radical-Polar Crossover C(sp³)–N Coupling via Inner-
Sphere Electron Shuttling
Y. Hong, C. Park, J. Jang, M. Oh, D. Kim, S. Lee* and S. Y. Hong*
Postdoctoral works at MIT
G. Li, Y. Kanda, S. Y. Hong and A. T. Radosevich*

Ph.D. works at KAIST
S. Kim, D. Kim, S. Y. Hong* and S. Chang* (*Co-corresponding authors)

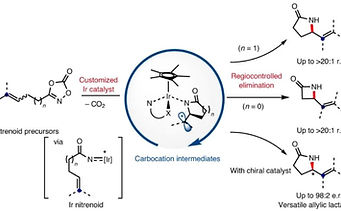
S. Y. Hong, D. Kim and S. Chang*
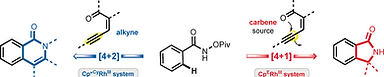
S. Huh,† S. Y. Hong,† and S. Chang*
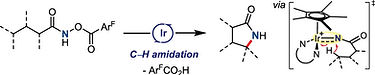
S. Y. Hong, J. Son, D. Kim, and S. Chang*
J. Am. Chem. Soc., 2018, 140, 12359-12363

S. Y. Hong,† Y. Park,† Y. Hwang, Y. Kim, M.-H. Baik,* and S. Chang*

S. Y. Hong,† J. Kwak,† and S. Chang*

bottom of page